Question – 1
I. Bond length of polar bonds is smaller than that of non-polar bondsII. Bond length is greater in alkane than in alkene and alkyneIII. The presence of lone pairs of electrons affects the bond angle
- A. I and III
- B. I only
- C. II only
- D. III only
- E. None of these
- Answer:E
- Answer Explanation:All the given statements are correct with respect to bond characteristics. The bond length of polar bonds is smaller than that of non-polar bonds. The carbon in alkane has sp3 hybridisation, carbon in alkene has sp2 hybridisation and carbon in alkyne has sp hybridisation. As the s-character is decreased, bond length is greater in alkane than in alkene and alkyne. The lone pairs of electrons always try to repel the bond pairs of electrons, so this affects the bond angle.
Question – 2
2. Determine the average life of U238 having t1/2 = 120 days.
- A. 202.02 days
- B. 143.5 days
- C. 365.4 days
- D. 173.1 days
- E. 150 days
- Answer:D
- Answer Explanation:t1/2 = 0.0693/?? = 0.693 X 1/?? (where, ?? = 1/??)t1/2 = 0.693 ????= t1/2 / 0.693= 120 / 0.693days= 173.16 days
Question – 3
3. Which of the following molecules has the highest boiling point?
- A. CH4
- B. CH3-F
- C. He2
- D. C2H5I
- E. C2H6
- Answer:D
- Answer Explanation:The boiling point of a substance depends upon the molecular weight or atomic size of the molecule. Helium, methane and ethane are smaller molecules than halogens. Among the halogens, atomic size of iodine is larger than that of fluorine. So, ethyl iodine has the highest boiling point.
Question – 4
4. Ca2+ is isoelectronic with
- A. Mn4+
- B. Ti3+
- C. Al3+
- D. Mg2+
- E. K+
- Answer:E
- Answer Explanation:Isoelectronic species is a set of species having same number of electrons. The atomic number or number of electrons forCa2+ is 18, Mn4+ is 21, Ti3+ is 19, Mg2+ is 10, K+ is 18. So, Ca2++ and K+ are isoelectronic species having equal number of 18 electrons.
Question – 5
5. Name the compound according to IUPAC nomenclature.
- A. 2-nitro-pentan-5-oic acid
- B. 2-nitro-butan-5-oic acid
- C. 2-nitro-butan-4-oic acid
- D. 4-nitropentanoic acid
- E. 3-nitrobutanoic acid
- Answer:D
- Answer Explanation:Among the nitro and acid groups, acid group gets the higher priority. So, numbering should be done in such a way that acid group gets lowest number. So, the name of this structure is 4-nitropentanoic acid.
Question – 6
6. At what pH will 9% of a compound with a pKa of 5.9 be in its basic form?
- A. 5.25
- B. 4.11
- C. 6.74
- D. 7.81
- E. 10.82
- Answer:D
- Answer Explanation:If 9% is in basic form, 91% is in acidic form. So, According to Henderson-Hasselbalch equation,pKa = pH + log [HA]/[A–]where, [HA] is concentration of compound in acidic form and [A–] is concentration of compound in basic form.pKa = pH + log [91/9]5.9 = pH + 1.91pKa = 7.81
Question – 7
7. The half life period for the first order reaction is expressed as
- A. t1/2 = k/2
- B. t1/2 = 0.693k
- C. t1/2 = 0.693 / k
- D. t1/2 = k / 0.693
- E. None of the above
- Answer:C
- Answer Explanation:Half life period of a reaction is defined as the time required to reduce the concentration of a reactant to one half of its initial value. Half life period for a first order reaction can be calculated using the following formula, which is derived from the first order rate law.t1/2 = 0.693 / k
Question – 8
8. What is the angle between the adjacent B-Cl bond lengths in BCl3 molecule?
- A. 900
- B. 450
- C. 1200
- D. 600
- E. 300
- Answer:C
- Answer Explanation:BCl3 is trigonal planar in shape. The bond angle between Cl-Cl is 1200.
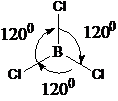
Question – 9
9. Calculate the Kc when a mixture containing 8.07 moles of H2 and 9.08 moles of I2 are reacted at 4480C until 13.38 moles of HI was formed at the equilibrium. (X=6.69 moles)
- A. 52.23
- B. 57.21
- C. 59.85
- D. 54.29
- E. 62.28
- Answer:D
- Answer Explanation:The reaction equilibrium is,
 Equilibrium constant,
Equilibrium constant,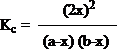 Where a=8.07 moles of H2, b=9.08 moles of I2, x=6.69 moles
Where a=8.07 moles of H2, b=9.08 moles of I2, x=6.69 moles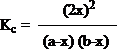 Kc= 54.29
Kc= 54.29
Question – 10
Ag, Ag+ || H+, H2(g)1atm, Pt. E0Ag+, Ag = 0.80 volts
- A. 0.9 V, not feasible.
- B. 1.8 V, feasible
- C. -1.9 V, not feasible
- D. 0 V, not feasible
- E. -0.8 V, not feasible
- Answer:E
- Answer Explanation:The standard reduction potential of Ag+, Ag is 0.80 VoltsThe standard EMF of the cell, E0E0cell = E0Right – E0LeftE0cell = [Std. Reduction potential of SHE] – [Std. Reduction potential of Ag+, Ag]The standard reduction potential of SHE is 0.Therefore, E0cell = 0 – (+0.8 V) = – 0.8 VSince, the E0cell is negative, the cell reaction is not feasible.
Question – 11
11. The hydrogen ion concentration of a fruit juice is 5.3 X 10-2 M. What is the pH of the juice?
- A. 1.27
- B. 1.48
- C. 1.77
- D. 2.00
- E. 2.23
- Answer:A
- Answer Explanation:pH = -log[H+]Given, [H+] = 5.3 X 10-2 MTherefore, pH = -log (5.3 X 10-2) = -(-1.27) =1.27
Question – 12
12. The order of stability of free radicals is
- A. CH3* > (CH3)3C* > (CH3)2 CH* > CH3CH2*
- B. CH3* > CH3CH2* > (CH3)2 CH* > (CH3)3C*
- C. (CH3)2 CH* > CH3CH2* > CH3* > (CH3)3C*
- D. (CH3)3C* > (CH3)2 CH* > CH3CH2* > CH3*
- E. CH3CH2* > (CH3)2 CH* > (CH3)3C* > CH3*
- Answer:D
- Answer Explanation:The groups which can donate electrons will stabilise the free radicals. So, the tertiary free radical has three methyl groups, which donate more electrons than secondary and primary. Also, the energy required to dissociate the bond is much lesser in tertiary free radical than in secondary, primary and ethyl groups. So, lesser the energy required, easier the formation of product. Thus, the tertiary free radical is more stable.
Question – 13
13. What is the reagent used for the synthesis of benzoic acid from toluene?
- A. HNO3
- B. LiAlH4
- C. H+/ KMnO4
- D. Zn/NaOH
- E. H+/ H2O
- Answer:C
- Answer Explanation:Toluene in the presence of a strong oxidising agent like KMnO4 and an acid catalyst, forms benzoic acid.
Question – 14
14. Calculate the coefficient of variance for the given titration values.13.5, 15.0, 9.5, 14.3, 12.6
- A. 55.05
- B. 32.08
- C. 37.56
- D. 33.00
- E. 18.59
- Answer:D
- Answer Explanation:The coefficient of variance can be calculated by using standard deviation values and mean values.The mean value for the given titration values = (13.5+15.0+9.5+14.3+12.6)/5 = 12.98Standard Deviation = (13.5-12.98)2+(15-12.98)2+(9.5-12.98)2+(14.3-12.98)2+(12.6-12.98)2=0.2704+4.0804+12.1104+1.7424+0.1444 = ???18.348 = 4.2834Coefficient of variance = (Standard deviation X 100) / MeanC.V = 4.2834 X 100) / 12.98 = 33.00
Question – 15
15. Which is the most stable conformation of cyclohexane?
- A. Chair form
- B. Boat form
- C. Twist boat form
- D. Twist chair form
- E. Both A and B
- Answer:A
- Answer Explanation:Cyclohexane is not a planar hexagon. So, it can exist preferentially in the chair conformation than in the less stable boat conformation, because in the chair conformation all the bonds are staggered. Therefore, the chair conformation is strain free compared to the other conformations.